Breakthroughs in Stopping Aging
Japanese researchers are making groundbreaking discoveries on the mechanisms of aging and working to apply them. As we age, senescent cells, or aged cells that have stopped dividing, accumulate, causing inflammation that can damage blood vessels and organs. Animal experiments have shown that removing these cells improves kidney function and reduces arteriosclerosis. They have led to the identification of a drug and development of a vaccine to eliminate the cells.

Transcript
Join us as we explore Medical Frontiers.
Humans have been in an eternal quest to be able to live healthy active lives into their 80s or 90s.
I'm 75. Getting old doesn't bother me much,
but approaching 80 makes me a bit anxious.
Younger individuals generally have superior physical abilities,
and as people age, their muscle strength gradually decreases.
This is believed to be one part of the aging process.
But this conventional wisdom may be overturned.
A recent study suggests that it may be possible to reverse the aging process.
Research on the topic is progressing and the mechanism behind it is becoming clearer.
In 2021, Japanese research groups published a series of groundbreaking discoveries
in international scientific journals that defied long-held beliefs.
This is an experiment comparing the muscle strength of two mice.
The mice were two years old, which is equivalent to about 60 to 70 years old in humans.
The mouse on the left gradually lost its ability to keep up with the rotating rod and fell off.
But the one on the right managed to hold on for 17 seconds longer.
The difference was that the mouse on the right had been injected with a drug identified by Japanese researchers
which removes cells that can cause age-related diseases.
Other aged mice injected with the same drug showed a significant improvement in their ability to hang onto a bar.
While mice that weren't given the drug could only hang on for an average of 30 seconds,
the treated mice were able to hang on for 100 seconds.
In a different experiment, researchers prepared two groups of mice with arteriosclerosis,
or hardening of the arteries due to plaque.
They fed both a high-fat diet, but injected one group with their newly developed vaccine that stops aging.
The mice in the control group had widespread plaque buildup, shown in the image in red.
However, the mice that received the vaccine had little plaque formation.
These findings have drawn global attention, sparking hope that one day, it will become possible to stop aging.
To learn more, we spoke with Minamino Tohru, a cardiologist who has been studying aging for over 30 years.
So what actually is aging in medical terms?
There are various definitions of aging.
One of them is based on age.
As we age, mortality naturally increases.
Some define this as aging.
Minamino's research on aging is not about changes in appearance, such as graying hair or wrinkles.
Even if people lead healthy lives, they develop various diseases in old age.
Minamino has been conducting research to find the underlying causes of such age-related diseases.
As we get older, our bodily functions
and capabilities decline.
The causes for this are being researched
extensively. One of them is cellular aging.
Our bodies are made up of about 60 trillion cells.
They undergo repeated divisions every day to sustain life.
Our bones, blood and organs stay healthy because our old cells are replaced by new ones.
However, all cells in humans, except for stem cells, can only divide 50 to 60 times.
After that, they stop dividing.
So do we know exactly why they stop dividing
or they, just after 50, 60 times, just give up - is there a reason why they stop dividing?
Various types of stress, such as oxidative stress
and ultraviolet rays, are believed to
directly damage our genes.
They're thought to cause cells to stop dividing.
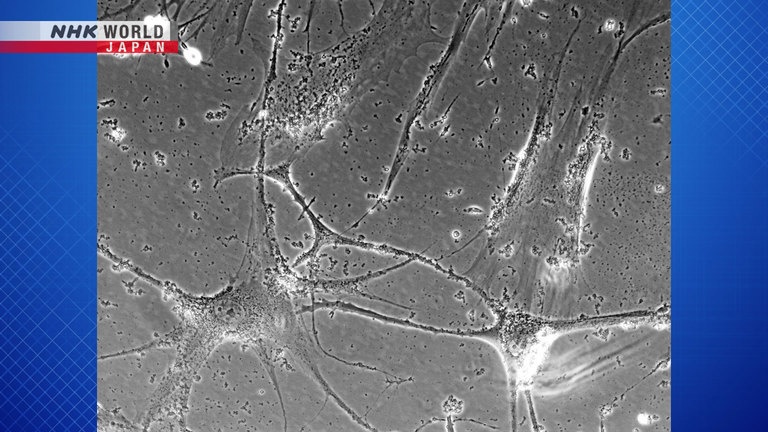
This image is of young cells undergoing division.
This one shows cells that have stopped dividing.
Each cell looks significantly elongated.
These cells that can no longer divide are called senescent cells.
Senescent cells exist in young people, even in babies.
But they are consumed by immune cells or undergo cell death on their own, allowing new cells to replace them.
However, as we age, senescent cells, which should be naturally eliminated from our bodies, survive and accumulate.
Recent studies have found that the buildup of senescent cells can cause a variety of age-related diseases.
Many researchers, including ourselves,
are beginning to understand that
in humans and in experimental animals
such as mice, senescent cells
are accumulating and causing harm.
And doctor, what research have you done specifically on senescent cells?
As a cardiologist, my focus is on heart attacks,
hardening of the arteries, and other conditions,
as well as the lifestyle-related diseases
that can cause them, such as diabetes.
I've been researching for several decades how
the accumulation of senescent cells is linked
to these diseases and how preventing their
accumulation could stave off or cure diseases.
Minamino's team examined this human coronary artery, which has developed arteriosclerosis.
The senescent cells have been stained in blue.
Compared with a healthy area, there is a much higher number of senescent cells in the affected region.
Various experiments using mice have shown that large numbers of senescent cells exist in organs affected by age-related diseases,
such as the eyes in glaucoma and cataracts and the brain in Alzheimer's disease and function of the kidneys.
Let's examine what accumulated senescent cells are doing inside our bodies.
Senescent cells tend to accumulate in
the arteries, the blood vessels that can
become blocked and cause heart attacks.
Oxidative stress and especially bad cholesterol
hurts the cells in the blood vessels
and causes them to become senescent.
This leads to inflammation and
eventually triggers arteriosclerosis.
The accumulated senescent cells that survive in organs release inflammatory substances, which harm healthy cells.
These inflammatory substances can cause various age-related diseases.
Would you say that all lifestyle diseases whether it be diabetes, cancer, or heart disease -
are they all related to senescent cells?
Most illnesses we treat, such as arteriosclerosis
and lifestyle diseases, are closely linked to
chronic inflammation. While it's not the sole
cause, the inflammatory molecules released by
senescent cells are believed to contribute
to the development of these diseases.
But why do we have this mechanism for cells to become senescent?
The system that halts cell division in senescent cells plays a significant role in our bodies.
It halts the growth of cancer cells.
When DNA is damaged, cells may
initiate an abnormal cell cycle
and start dividing uncontrollably.
This eventually leads to cancer.
To prevent that, cells deliberately
activate the aging process.
When cells begin to divide abnormally, like with cancer cells,
they activate a system that stops their own growth, preventing further divisions.
This leads to cell aging.
The process helps to protect us from developing cancer.
The cellular senescence mechanism is
our ultimate weapon against cancer.
Researchers around the world have been working on the challenge of how to safely remove senescent cells,
which have both positive and negative characteristics.
In 2021, a Japanese research team made an announcement that surprised the world.
The cellular senescence system itself does not
cause harm, but the cells it produces do.
We should develop technology or drugs that
kill those cells without affecting normal cells.
A team led by Nakanishi Makoto identified that a medicine can remove only senescent cells,
while leaving normal cells almost unharmed.
Nakanishi hypothesized that senescent cells manage to survive by producing a unique substance
that is not found in normal cells.
To explore this idea, his team spent five years diligently screening the genes of cells for this special substance.
The team discovered an enzyme called GLS-1, which turned out to be the key to the survival of senescent cells.
The GLS-1 enzyme is essential for producing glutamic acid, which serves as a source of energy for cells.
Nakanishi discovered that senescent cells consume more GLS-1 than regular cells.
We found that the GLS-1 enzyme is crucial
to the survival of senescent cells.
This led to the idea that we could eliminate
the cells by inhibiting the enzyme's activity.
This breakthrough led to the identification of GLS-1 inhibitor, a drug that suppresses the enzyme's activity.
The research team added GLS-1 inhibitor to senescent cells and observed the changes.
This image shows the senescent cells 24 hours later.
The significantly enlarged cells have disappeared.
Furthermore, the team also discovered how the GLS-1 enzyme works to support the survival of senescent cells.
We found that senescent cells were using
GLS-1 to produce ammonia in order to survive.
Human cells contain a structure called the lysosome that breaks down cellular waste and foreign substances.
Inside the lysosome are powerful acidic substances.
When a cell undergoes aging, the membrane of its lysosome breaks, causing the acidic substances to leak out.
As a result, the cell becomes acidic and dies.
However, when senescent cells use GLS-1 to produce their energy source,
glutamic acids, from glutamine, they simultaneously generate ammonia.
Because ammonia is alkaline, it neutralizes the acidic substances that have leaked out from the lysosomes.
This prevents the cells from becoming acidic, allowing them to survive.
When GLS-1 inhibitor enters senescent cells, the GLS-1 enzyme stops working, and the production of ammonia ceases.
As a result, the senescent cells start to die off.
Nakanishi verified the effectiveness of GLS-1 inhibitor by comparing aged mice
that received it with those that didn't receive it.
He placed them on spinning rods to see how long they could hold on.
The mice that had their senescent cells removed were able to stay on the rod for 10 to 20 seconds longer than the other group.
The removal of senescent cells also led to improvements in various diseases that were present in the aged mice.
The left image shows the aorta of a mouse with arteriosclerosis.
The red areas indicate where the hardening has occurred.
In the right image of the mouse that has had its senescent cells removed,
there are hardly any red areas, suggesting an improvement in arteriosclerosis.
Changes were also observed in the kidneys.
This is an image of a glomerulus, a structure in the kidney responsible for filtering waste and sodium from the blood.
In aged mice, they are tight and stiff, indicating a decline in filtering ability.
But in aged mice that had their senescent cells removed,
they became softer and developed gaps, and kidney function recovered.
The functions of the lungs also decline with age.
One of the reasons for this is fibrosis, a thickening or scarring of tissue, indicated by the blue-stained areas.
After the drug was administered, the fibrosis improved, and lung function was restored.
Senescent cells build up in
various parts of the body.
We believe that by removing them, we can improve
the declining functions of organs and tissues.
The drug Nakanishi identified is undergoing clinical trials in the United States for other diseases.
So far, it has been proven safe for human use.
In the past, there have been reports
of drugs that can kill senescent cells.
But they all had relatively strong side effects.
The one we've identified has
mild side effects and is gentle on cells.
We believe it can be put to practical use.
Minamino discovered a different method to eliminate senescent cells without harming normal cells.
In 2021, his study results were published in "Nature Aging," stunning researchers worldwide.
His method involves a vaccine.
Our method of targeting senescent cells
utilizes the body's immune system.
The immune system is supposed to remove
senescent cells, but it doesn't always succeed.
Our method gives a boost to the system
to facilitate the removal of the cells.
White blood cells, a part of our immune system, have the ability to attack and remove senescent cells.
Minamino's team found a unique marker present on senescent cells.
The team developed a vaccine that effectively guides white blood cells to recognize the marker and connect to it.
When they tested the vaccine, the white blood cells did as expected.
They started attacking the senescent cells, causing them to die.
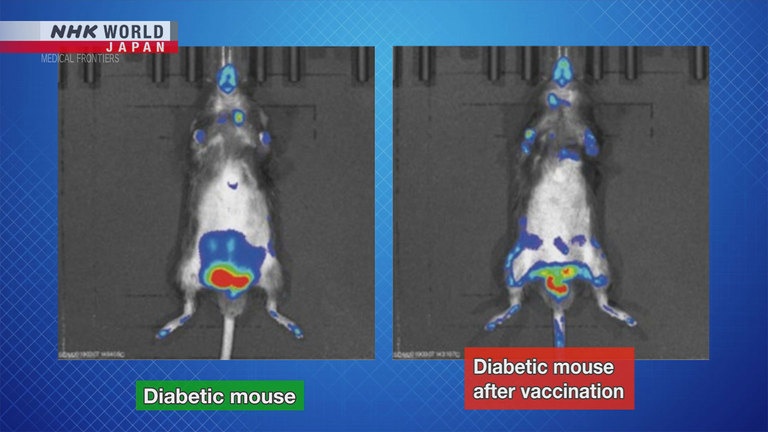
Minamino injected the vaccine into diabetic middle-aged mice,
which would be equivalent to around 50 years of age in humans.
After the vaccination, the mice's senescent cells, shown in red, decreased significantly.
Their post-meal blood sugar level improved, and their insulin level was restored.
The vaccine also led to a reduction in senescent cells in mice with arteriosclerosis.
Plaque that had accumulated in the blood vessels almost disappeared.
And in terms of the antibody vaccination,
how many years or how long will it take for this to be into practice so that, you know,
that I can at, you know, a ripe old age be able to have this?
We would like to put it to practical use
as soon as possible.
But there are several challenges,
such as determining who will
be eligible for the vaccine.
In addition, aging is not recognized as
a disease, so we cannot treat patients for it.
To start clinical studies of drugs or vaccines on humans, doctors must specify the disease they intend to treat.
However, the accumulation of senescent cells is not recognized as a disease.
Do you think there will ever be a stage that we can achieve immortality?
No, I don't think that's likely. The maximum
lifespan of humans is probably around 120 years.
I think it is predetermined
and varies among different species.
That's the hypothesis that I believe.
My treatment aims for healthy aging,
rather than extending the maximum
lifespan or achieving immortality.
I think the key thing you mentioned is living that healthy, active life,
the goal of lifespan is the goal of having a healthy, active lifespan.
That's a very important point.
No one wants to spend the last
10 years of their lives bedridden.
I believe it's crucial to intervene to prevent
that by keeping people healthy.
Finally, we asked Minamino what we can do to prevent the accumulation of senescent cells.
I eat a very light dinner. As a result,
I end up fasting from evening until morning.
Excessive eating can lead to weight gain and
a buildup of senescent cells in belly fat.
Fasting creates a certain level of hunger, which
is said to prevent these cells from accumulating.
I believe this is important for our health.
Experiments with mice have found that fasting can suppress cellular aging.
But Minamino recommends a more moderate approach.
He suggests reducing the amount we eat at night, so that we will feel hungry when we wake up in the morning.
Socializing is another key to slower aging.
In an interesting study, researchers attached
the skin of a young mouse to that of an aged one,
and their blood started circulating between them.
The young mouse aged slightly,
while the aged one became a bit younger.
This result was consistently observed.
As this study suggests, I believe it's crucial to
interact with people from other generations.
In my department, there are people in their 20s,
30s and 40s. Many of them are very young.
Their perspectives and life experiences
differ significantly from mine,
so I find it fascinating to interact with
these colleagues from different generations.
Listening to them makes me feel
more energized and uplifted.
My granddaughter was born last week.
Congratulations, oh my gosh, first grandchild?
Yes.
I want to contribute to children as well.
Thank you.
Many countries have super-aged populations.
Many people worry about whether they can remain healthy until the end, and the high costs of medical care.
If the process of aging can be reversed or delayed, it may become possible to postpone age-related diseases.
Research on aging is advancing from multiple perspectives,
aiming for a future where we can live out our later years happily, and in good health.